
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Drug/Regimen
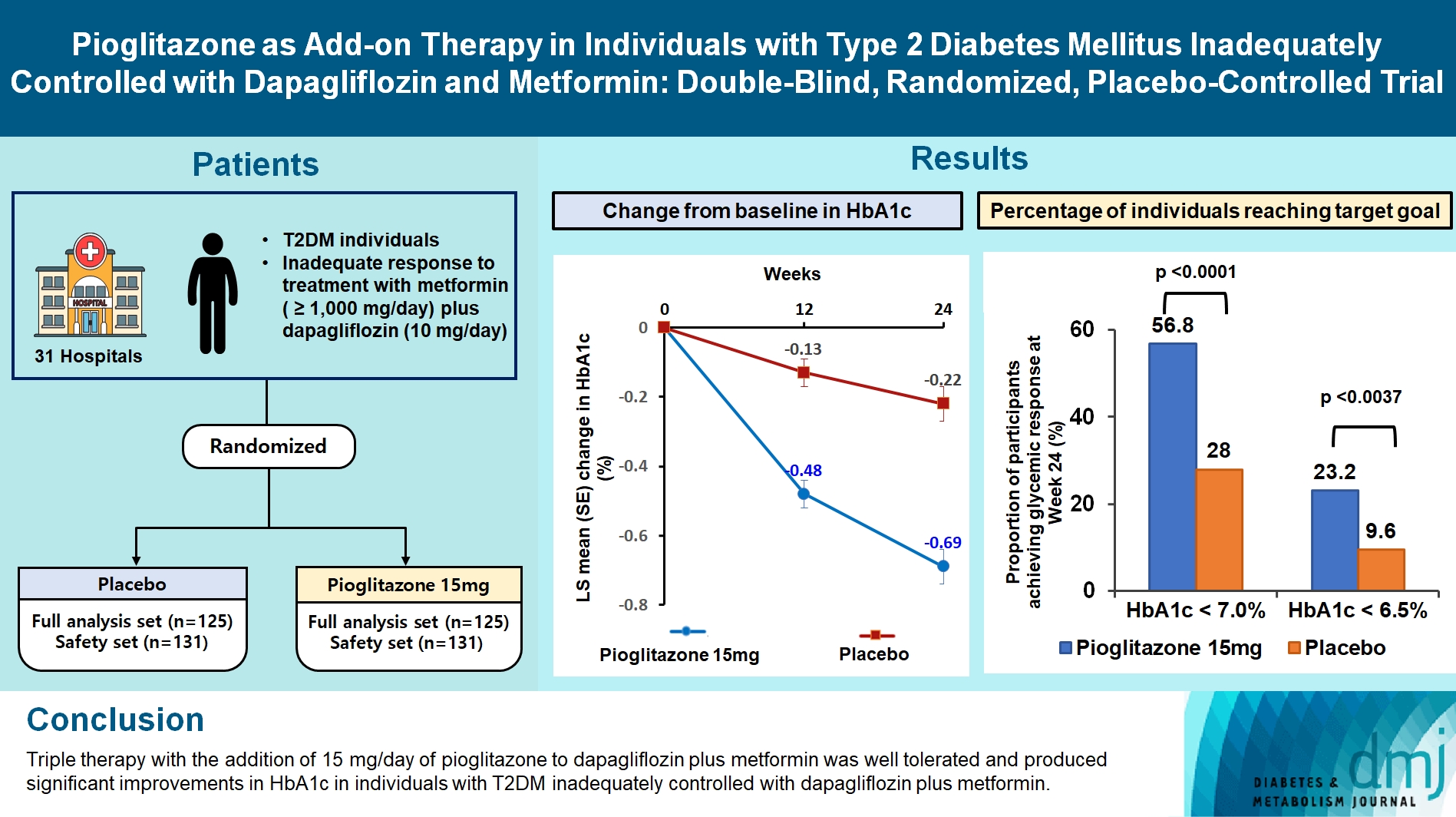
- Pioglitazone as Add-on THERAPY in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial
- Ji Hye Heo, Kyung Ah Han, Jun Hwa Hong, Hyun-Ae Seo, Eun-Gyoung Hong, Jae Myung Yu, Hye Seung Jung, Bong-Soo Cha
- Received September 1, 2023 Accepted October 25, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0314 [Epub ahead of print]
- 1,241 View
- 127 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study assessed the efficacy and safety of triple therapy with pioglitazone 15 mg add-on versus placebo in patients with type 2 diabetes mellitus (T2DM) inadequately controlled with metformin and dapagliflozin.
Methods
In this multicenter, double-blind, randomized, phase 3 study, patients with T2DM with an inadequate response to treatment with metformin (≥1,000 mg/day) plus dapagliflozin (10 mg/day) were randomized to receive additional pioglitazone 15 mg/day (n=125) or placebo (n=125) for 24 weeks. The primary endpoint was the change in glycosylated hemoglobin (HbA1c) levels from baseline to week 24 (ClinicalTrials.gov identifier: NCT05101135).
Results
At week 24, the adjusted mean change from baseline in HbA1c level compared with placebo was significantly greater with pioglitazone treatment (–0.47%; 95% confidence interval, –0.61 to –0.33; P<0.0001). A greater proportion of patients achieved HbA1c <7% or <6.5% at week 24 with pioglitazone compared to placebo as add-on to 10 mg dapagliflozin and metformin (56.8% vs. 28% for HbA1c <7%, and 23.2% vs. 9.6% for HbA1c <6.5%; P<0.0001 for all). The addition of pioglitazone also significantly improved triglyceride, highdensity lipoprotein cholesterol levels, and homeostatic model assessment of insulin resistance levels, while placebo did not. The incidence of treatment-emergent adverse events was similar between the groups, and the incidence of fluid retention-related side effects by pioglitazone was low (1.5%).
Conclusion
Triple therapy with the addition of 15 mg/day of pioglitazone to dapagliflozin plus metformin was well tolerated and produced significant improvements in HbA1c in patients with T2DM inadequately controlled with dapagliflozin plus metformin.
- Drug Regimen
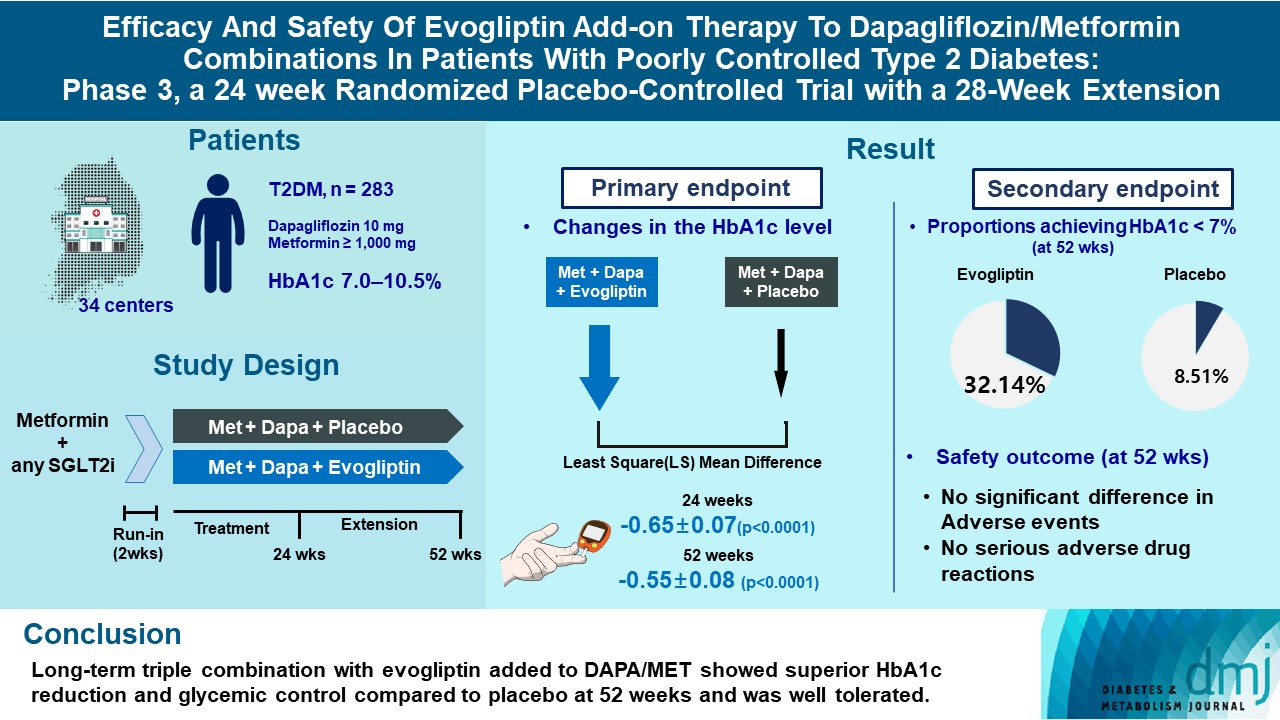
- Efficacy and Safety of Evogliptin Add-on Therapy to Dapagliflozin/Metformin Combinations in Patients with Poorly Controlled Type 2 Diabetes Mellitus: A 24-Week Multicenter Randomized Placebo-Controlled Parallel-Design Phase-3 Trial with a 28-Week Extension
- Jun Sung Moon, Il Rae Park, Hae Jin Kim, Choon Hee Chung, Kyu Chang Won, Kyung Ah Han, Cheol-Young Park, Jong Chul Won, Dong Jun Kim, Gwan Pyo Koh, Eun Sook Kim, Jae Myung Yu, Eun-Gyoung Hong, Chang Beom Lee, Kun-Ho Yoon
- Diabetes Metab J. 2023;47(6):808-817. Published online September 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0387
- 2,610 View
- 283 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigates the long-term efficacy and safety of evogliptin add-on therapy in patients with inadequately controlled type 2 diabetes mellitus (T2DM) previously received dapagliflozin and metformin (DAPA/MET) combination.
Methods
In this multicenter randomized placebo-controlled phase 3 trial, patients with glycosylated hemoglobin (HbA1c) levels 7.0% to 10.5% (n=283) previously used DAPA 10 mg plus MET (≥1,000 mg) were randomly assigned to the evogliptin 5 mg once daily or placebo group (1:1). The primary endpoint was the difference in the HbA1c level from baseline at week 24, and exploratory endpoints included the efficacy and safety of evogliptin over 52 weeks (trial registration: ClinicalTrials.gov NCT04170998).
Results
Evogliptin add-on to DAPA/MET therapy was superior in HbA1c reduction compared to placebo at weeks 24 and 52 (least square [LS] mean difference, –0.65% and –0.55%; 95% confidence interval [CI], –0.79 to –0.51 and –0.71 to –0.39; P<0.0001). The proportion of patients achieving HbA1c <7% was higher in the triple combination group at week 52 (32.14% vs. 8.51% in placebo; odds ratio, 5.62; P<0.0001). Evogliptin significantly reduced the fasting glucose levels and mean daily glucose levels with improvement in homeostatic model assessment of β-cell function (LS mean difference, 9.04; 95% CI, 1.86 to 16.21; P=0.0138). Adverse events were similar between the groups, and no serious adverse drug reactions were reported in the evogliptin group.
Conclusion
Long-term triple combination with evogliptin added to DAPA/MET showed superior HbA1c reduction and glycemic control compared to placebo at 52 weeks and was well tolerated.
- Drug/Regimen
- A Real-World Study of Long-Term Safety and Efficacy of Lobeglitazone in Korean Patients with Type 2 Diabetes Mellitus
- Bo-Yeon Kim, Hyuk-Sang Kwon, Suk Kyeong Kim, Jung-Hyun Noh, Cheol-Young Park, Hyeong-Kyu Park, Kee-Ho Song, Jong Chul Won, Jae Myung Yu, Mi Young Lee, Jae Hyuk Lee, Soo Lim, Sung Wan Chun, In-Kyung Jeong, Choon Hee Chung, Seung Jin Han, Hee-Seok Kim, Ju-Young Min, Sungrae Kim
- Diabetes Metab J. 2022;46(6):855-865. Published online March 8, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0264
- 6,723 View
- 298 Download
- 6 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Thiazolidinediones (TZDs) have been associated with various safety concerns including weight gain, bladder cancer, and congestive heart failure (CHF). This study evaluated the efficacy and safety of lobeglitazone, a novel TZD in patients with type 2 diabetes mellitus (T2DM) in real practice.
Methods
In this non-interventional, multi-center, retrospective, and observational study conducted at 15 tertiary or secondary referral hospitals in Korea, a total of 2,228 patients with T2DM who received lobeglitazone 0.5 mg for more than 1 year were enrolled.
Results
Overall adverse events (AEs) occurred in 381 patients (17.10%) including edema in 1.97% (n=44). Cerebrovascular and cardiovascular diseases were identified in 0.81% (n=18) and 0.81% (n=18), respectively. One case of CHF was reported as an AE. Edema occurred in 1.97% (n=44) of patients. Hypoglycemia occurred in 2.47% (n=55) of patients. Fracture occurred in 1.17% (n=26) of all patients. Lobeglitazone significantly decreased HbA1c level, resulting in a mean treatment difference of -1.05%± 1.35% (P<0.001), and decreased total cholesterol, triglyceride, and low-density lipoprotein cholesterol. However, it increased high-density lipoprotein cholesterol, regardless of statin administration. The patients who received lobeglitazone 0.5 mg showed an apparent reduction in glycosylated hemoglobin (HbA1c) from baseline during the first 6 months of treatment. The HbA1c levels remained stable between months 6 and 42.
Conclusion
Lobeglitazone has long-term safety profile, good glycemic-lowering effect and long-term durability of glycemic control in real-world clinical settings. -
Citations
Citations to this article as recorded by- Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis
Deep Dutta, Saptarshi Bhattacharya, Manoj Kumar, Priyankar K. Datta, Ritin Mohindra, Meha Sharma
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102697. CrossRef - Efficacy and safety of lobeglitazone, a new Thiazolidinedione, as compared to the standard of care in type 2 diabetes mellitus: A systematic review and meta-analysis
Shashank R. Joshi, Saibal Das, Suja Xaviar, Shambo Samrat Samajdar, Indranil Saha, Sougata Sarkar, Shatavisa Mukherjee, Santanu Kumar Tripathi, Jyotirmoy Pal, Nandini Chatterjee
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102703. CrossRef - Will lobeglitazone rival pioglitazone? A systematic review and critical appraisal
Kalyan Kumar Gangopadhyay, Awadhesh Kumar Singh
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(4): 102747. CrossRef - Lobeglitazone
Reactions Weekly.2023; 1948(1): 262. CrossRef - Lobeglitazone, a novel thiazolidinedione, for secondary prevention in patients with ischemic stroke: a nationwide nested case-control study
Joonsang Yoo, Jimin Jeon, Minyoul Baik, Jinkwon Kim
Cardiovascular Diabetology.2023;[Epub] CrossRef - Lobeglitazone and Its Therapeutic Benefits: A Review
Balamurugan M, Sarumathy S, Robinson R
Cureus.2023;[Epub] CrossRef - Oldies but Goodies: Thiazolidinedione as an Insulin Sensitizer with Cardioprotection
Eun-Hee Cho
Diabetes & Metabolism Journal.2022; 46(6): 827. CrossRef
- Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis
- Drug/Regimen
- Efficacy and Safety of Omega-3 Fatty Acids in Patients Treated with Statins for Residual Hypertriglyceridemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
- Ji Eun Jun, In-Kyung Jeong, Jae Myung Yu, Sung Rae Kim, In Kye Lee, Kyung-Ah Han, Sung Hee Choi, Soo-Kyung Kim, Hyeong Kyu Park, Ji-Oh Mok, Yong-ho Lee, Hyuk-Sang Kwon, So Hun Kim, Ho-Cheol Kang, Sang Ah Lee, Chang Beom Lee, Kyung Mook Choi, Sung-Ho Her, Won Yong Shin, Mi-Seung Shin, Hyo-Suk Ahn, Seung Ho Kang, Jin-Man Cho, Sang-Ho Jo, Tae-Joon Cha, Seok Yeon Kim, Kyung Heon Won, Dong-Bin Kim, Jae Hyuk Lee, Moon-Kyu Lee
- Diabetes Metab J. 2020;44(1):78-90. Published online June 20, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0265
- 9,326 View
- 190 Download
- 7 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Cardiovascular risk remains increased despite optimal low density lipoprotein cholesterol (LDL-C) level induced by intensive statin therapy. Therefore, recent guidelines recommend non-high density lipoprotein cholesterol (non-HDL-C) as a secondary target for preventing cardiovascular events. The aim of this study was to assess the efficacy and tolerability of omega-3 fatty acids (OM3-FAs) in combination with atorvastatin compared to atorvastatin alone in patients with mixed dyslipidemia.
Methods This randomized, double-blind, placebo-controlled, parallel-group, and phase III multicenter study included adults with fasting triglyceride (TG) levels ≥200 and <500 mg/dL and LDL-C levels <110 mg/dL. Eligible subjects were randomized to ATOMEGA (OM3-FAs 4,000 mg plus atorvastatin calcium 20 mg) or atorvastatin 20 mg plus placebo groups. The primary efficacy endpoints were the percent changes in TG and non-HDL-C levels from baseline at the end of treatment.
Results After 8 weeks of treatment, the percent changes from baseline in TG (−29.8% vs. 3.6%,
P <0.001) and non-HDL-C (−10.1% vs. 4.9%,P <0.001) levels were significantly greater in the ATOMEGA group (n =97) than in the atorvastatin group (n =103). Moreover, the proportion of total subjects reaching TG target of <200 mg/dL in the ATOMEGA group was significantly higher than that in the atorvastatin group (62.9% vs. 22.3%,P <0.001). The incidence of adverse events did not differ between the two groups.Conclusion The addition of OM3-FAs to atorvastatin improved TG and non-HDL-C levels to a significant extent compared to atorvastatin alone in subjects with residual hypertriglyceridemia.
-
Citations
Citations to this article as recorded by- Association Between Omega‐3 Fatty Acid Intake and Dyslipidemia: A Continuous Dose–Response Meta‐Analysis of Randomized Controlled Trials
Tianjiao Wang, Xin Zhang, Na Zhou, Yuxuan Shen, Biao Li, Bingshu E. Chen, Xinzhi Li
Journal of the American Heart Association.2023;[Epub] CrossRef - Nutraceutical support in the prevention and treatment of cardiovascular diseases
E. V. Gracheva, E. A. Starovoytova, E. S. Kulikov, N. A. Kirillova, S. V. Fedosenko, M. A. Balaganskaya, D. V. Kromka
Rational Pharmacotherapy in Cardiology.2023; 19(3): 298. CrossRef - Effect of coadministration of omega-3 fatty acids with glimepiride on glycemic control, lipid profile, irisin, and sirtuin-1 in type 2 diabetes mellitus patients: a randomized controlled trial
Rehab H. Werida, Aalaa Ramzy, Youssri Nassief Ebrahim, Maged Wasfy Helmy
BMC Endocrine Disorders.2023;[Epub] CrossRef - The Effect of Dietary Interventions on Hypertriglyceridemia: From Public Health to Molecular Nutrition Evidence
Karla Paulina Luna-Castillo, Xochitl Citlalli Olivares-Ochoa, Rocío Guadalupe Hernández-Ruiz, Iris Monserrat Llamas-Covarrubias, Saraí Citlalic Rodríguez-Reyes, Alejandra Betancourt-Núñez, Barbara Vizmanos, Erika Martínez-López, José Francisco Muñoz-Valle
Nutrients.2022; 14(5): 1104. CrossRef - The effect of omega-3 fatty acids and its combination with statins on lipid profile in patients with hypertriglyceridemia: A systematic review and meta-analysis of randomized controlled trials
Yunjiao Yang, Wen Deng, Yanmei Wang, Tongyi Li, Yiding Chen, Cong Long, Qing Wen, Yue Wu, Qiu Chen
Frontiers in Nutrition.2022;[Epub] CrossRef - Comparison of the Efficacy and Safety of Atorvastatin 40 mg/ω-3 Fatty Acids 4 g Fixed-dose Combination and Atorvastatin 40 mg Monotherapy in Hypertriglyceridemic Patients who Poorly Respond to Atorvastatin 40 mg Monotherapy: An 8-week, Multicenter, Random
Jong Shin Woo, Soon Jun Hong, Dong Hoon Cha, Kee Sik Kim, Moo Hyun Kim, Jun-Won Lee, Myung Ho Jeong, Jin-Ok Jeong, Jun-Hee Lee, Doo Soo Jeon, Eun Joo Cho, Soon Kil Kim, Jun Kwan, Chang Gyu Park, Hae Young Lee, Taek Jong Hong, Jinho Shin, Ho Joong Youn, Do
Clinical Therapeutics.2021; 43(8): 1419. CrossRef - All-Cause Mortality and Cardiovascular Death between Statins and Omega-3 Supplementation: A Meta-Analysis and Network Meta-Analysis from 55 Randomized Controlled Trials
Jeongseon Kim, Tung Hoang, Ji-Myung Kim, So Young Bu, Jeong-Hwa Choi, Eunju Park, Seung-Min Lee, Eunmi Park, Ji Yeon Min, In Seok Lee, So Young Youn, Jee-Young Yeon
Nutrients.2020; 12(10): 3203. CrossRef
- Association Between Omega‐3 Fatty Acid Intake and Dyslipidemia: A Continuous Dose–Response Meta‐Analysis of Randomized Controlled Trials
- Obesity and Metabolic Syndrome
- The Association between Z-Score of Log-Transformed A Body Shape Index and Cardiovascular Disease in Korea
- Wankyo Chung, Jung Hwan Park, Hye Soo Chung, Jae Myung Yu, Shinje Moon, Dong Sun Kim
- Diabetes Metab J. 2019;43(5):675-682. Published online April 26, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0169
- 7,755 View
- 59 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background In order to overcome the limitations of body mass index (BMI) and waist circumference (WC), the z-score of the log-transformed A Body Shape Index (LBSIZ) has recently been introduced. In this study, we analyzed the relationship between the LBSIZ and cardiovascular disease (CVD) in a Korean representative sample.
Methods Data were collected from the Korea National Health and Nutrition Examination VI to V. The association between CVD and obesity indices was analyzed using a receiver operating characteristic curve. The cut-off value for the LBSIZ was estimated using the Youden index, and the odds ratio (OR) for CVD was determined via multivariate logistic regression analysis. ORs according to the LBSIZ value were analyzed using restricted cubic spline regression plots.
Results A total of 31,227 Korean healthy adults were analyzed. Area under the curve (AUC) of LBSIZ against CVD was 0.686 (95% confidence interval [CI], 0.671 to 0.702), which was significantly higher than the AUC of BMI (0.583; 95% CI, 0.567 to 0.599) or WC (0.646; 95% CI, 0.631 to 0.661) (
P <0.001). Similar results were observed for stroke and coronary artery diseases. The cut-off value for the LBSIZ was 0.35 (sensitivity, 64.5%; specificity, 64%; OR, 1.29, 95% CI, 1.12 to 1.49). Under restricted cubic spline regression, LBSIZ demonstrated that OR started to increase past the median value.Conclusion The findings of this study suggest that the LBSIZ might be more strongly associated with CVD risks compared to BMI or WC. These outcomes would be helpful for CVD risk assessment in clinical settings, especially the cut-off value of the LBSIZ suggested in this study.
-
Citations
Citations to this article as recorded by- Body Shape Index and Cardiovascular Risk in Individuals With Obesity
Nazlı Hacıağaoğlu, Can Öner, Hüseyin Çetin, Engin Ersin Şimşek
Cureus.2022;[Epub] CrossRef - Association between body shape index and risk of mortality in the United States
Heysoo Lee, Hye Soo Chung, Yoon Jung Kim, Min Kyu Choi, Yong Kyun Roh, Wankyo Chung, Jae Myung Yu, Chang-Myung Oh, Shinje Moon
Scientific Reports.2022;[Epub] CrossRef - Utility of the Z-score of log-transformed A Body Shape Index (LBSIZ) in the assessment for sarcopenic obesity and cardiovascular disease risk in the United States
Wankyo Chung, Jung Hwan Park, Hye Soo Chung, Jae Myung Yu, Dong Sun Kim, Shinje Moon
Scientific Reports.2019;[Epub] CrossRef
- Body Shape Index and Cardiovascular Risk in Individuals With Obesity
- Clinical Diabetes & Therapeutics
- Association between Serum Selenium Level and the Presence of Diabetes Mellitus: A Meta-Analysis of Observational Studies
- Juno Kim, Hye Soo Chung, Min-Kyu Choi, Yong Kyun Roh, Hyung Joon Yoo, Jung Hwan Park, Dong Sun Kim, Jae Myung Yu, Shinje Moon
- Diabetes Metab J. 2019;43(4):447-460. Published online January 2, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0123
- 6,303 View
- 98 Download
- 35 Web of Science
- 35 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Epidemiological studies have suggested an association between selenium (Se) and diabetes mellitus (DM). However, different studies have reported conflicting results. Therefore, we performed a comprehensive meta-analysis to clarify the impact of Se on DM.
Methods We searched the PubMed database for studies on the association between Se and DM from inception to June 2018.
Results Twenty articles evaluating 47,930 participants were included in the analysis. The meta-analysis found that high levels of Se were significantly associated with the presence of DM (pooled odds ratios [ORs], 1.88; 95% confidence interval [CI], 1.44 to 2.45). However, significant heterogeneity was found (
I2 =82%). Subgroup analyses were performed based on the Se measurement methods used in each study. A significant association was found between high Se levels and the presence of DM in the studies that used blood (OR, 2.17; 95% CI, 1.60 to 2.93;I2 =77%), diet (OR, 1.61; 95% CI, 1.10 to 2.36;I2 =0%), and urine (OR, 1.49; 95% CI, 1.02 to 2.17;I2 =0%) as samples to estimate Se levels, but not in studies on nails (OR, 1.24; 95% CI, 0.52 to 2.98;I2 =91%). Because of significant heterogeneity in the studies with blood, we conducted a sensitivity analysis and tested the publication bias. The results were consistent after adjustment based on the sensitivity analysis as well as the trim and fill analysis for publication bias.Conclusion This meta-analysis demonstrates that high levels of Se are associated with the presence of DM. Further prospective and randomized controlled trials are warranted to elucidate the link better.
-
Citations
Citations to this article as recorded by- Increased Expression of PHGDH Under High-Selenium Stress In Vivo
Qin Wang, Jianrong Wang, Xue Zhang, Yiqun Liu, Feng Han, Xuesong Xiang, Yanbin Guo, Zhen-wu Huang
Biological Trace Element Research.2024;[Epub] CrossRef - Dosage-effect of selenium supplementation on blood glucose and oxidative stress in type 2 diabetes mellitus and normal mice
Xiaxia Cai, Zhuo Hu, Mingyuan Zhang, Qinyu Dang, Qian Yang, Xiaoyan Zhao, Yandi Zhu, Yadi Zhang, Yuchen Wei, Haiqin Fang, Huanling Yu
Journal of Trace Elements in Medicine and Biology.2024; 83: 127410. CrossRef - Immunomodulation through Nutrition Should Be a Key Trend in Type 2 Diabetes Treatment
Katarzyna Napiórkowska-Baran, Paweł Treichel, Marta Czarnowska, Magdalena Drozd, Kinga Koperska, Agata Węglarz, Oskar Schmidt, Samira Darwish, Bartłomiej Szymczak, Zbigniew Bartuzi
International Journal of Molecular Sciences.2024; 25(7): 3769. CrossRef - Biological Activity of Selenium and Its Impact on Human Health
Giuseppe Genchi, Graziantonio Lauria, Alessia Catalano, Maria Stefania Sinicropi, Alessia Carocci
International Journal of Molecular Sciences.2023; 24(3): 2633. CrossRef - The Role of Selenium and Manganese in the Formation, Diagnosis and Treatment of Cervical, Endometrial and Ovarian Cancer
Anna Golara, Mateusz Kozłowski, Paweł Guzik, Sebastian Kwiatkowski, Aneta Cymbaluk-Płoska
International Journal of Molecular Sciences.2023; 24(13): 10887. CrossRef - Association of Selenium Intake and Selenium Concentrations with Risk of Type 2 Diabetes in Adults: A Narrative Review
Maha Alharithy, Nora Alafif
Metabolites.2023; 13(6): 767. CrossRef - Selenium-Containing Organic Fertilizer Application Affects Yield, Quality, and Distribution of Selenium in Wheat
Peng Chen, Hiba Shaghaleh, Yousef Alhaj Hamoud, Jing Wang, Wenxia Pei, Xianfu Yuan, Jianjian Liu, Cece Qiao, Wenhui Xia, Jianfei Wang
Life.2023; 13(9): 1849. CrossRef - Selenium Species in Diabetes Mellitus Type 2
Krystyna Pyrzynska, Aleksandra Sentkowska
Biological Trace Element Research.2023;[Epub] CrossRef - A Comprehensive Review on Selenium and Its Effects on Human Health and Distribution in Middle Eastern Countries
Marek Kieliszek, Iqra Bano, Hamed Zare
Biological Trace Element Research.2022; 200(3): 971. CrossRef - Selenium and clarithromycin loaded PLA-GO composite wound dressings by electrospinning method
Fatih Ciftci, Sumeyra Ayan, Nilüfer Duygulu, Yasemin Yilmazer, Zeynep Karavelioglu, Meyrem Vehapi, Rabia Cakır Koc, Mustafa Sengor, Hakan Yılmazer, Didem Ozcimen, Oguzhan Gunduz, Cem Bulent Ustundag
International Journal of Polymeric Materials and Polymeric Biomaterials.2022; 71(12): 898. CrossRef - The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities
Holger Steinbrenner, Leonidas H. Duntas, Margaret P. Rayman
Redox Biology.2022; 50: 102236. CrossRef - The Role of Selenium in Pathologies: An Updated Review
Giulia Barchielli, Antonella Capperucci, Damiano Tanini
Antioxidants.2022; 11(2): 251. CrossRef - Selenium and Selenoproteins at the Intersection of Type 2 Diabetes and Thyroid Pathophysiology
Francesca Gorini, Cristina Vassalle
Antioxidants.2022; 11(6): 1188. CrossRef - Higher selenium was associated with higher risk of diabetes: Consistent evidence from longitudinal and cross-sectional studies based on nail and serum selenium measures
Ranqi Shao, Liqin Su, Li Li, Jinghuan Wu, Xiaohong He, Deqian Mao, Yibin Cheng, Jingyi Liu, Chen Chen, Yinlong Jin, Sujuan Gao
Science of The Total Environment.2022; 840: 156618. CrossRef - The Roles and Pathogenesis Mechanisms of a Number of Micronutrients in the Prevention and/or Treatment of Chronic Hepatitis, COVID-19 and Type-2 Diabetes Mellitus
Khalid M. Sumaily
Nutrients.2022; 14(13): 2632. CrossRef - Associations between Circulating SELENOP Level and Disorders of Glucose and Lipid Metabolism: A Meta-Analysis
Ruirui Yu, Zhoutian Wang, Miaomiao Ma, Ping Xu, Longjian Liu, Alexey A. Tinkov, Xin Gen Lei, Ji-Chang Zhou
Antioxidants.2022; 11(7): 1263. CrossRef - Cross-Sectional Association of Blood Selenium with Glycemic Biomarkers among U.S. Adults with Normoglycemia in the National Health and Nutrition Examination Survey 2013–2016
Jingli Yang, En Chen, Cheukling Choi, Kayue Chan, Qinghua Yang, Juwel Rana, Bo Yang, Chuiguo Huang, Aimin Yang, Kenneth Lo
Nutrients.2022; 14(19): 3972. CrossRef - Plasma and vitreous selenium concentrations in patients with type 2 diabetes and diabetic retinopathy
Chunmiao Wang, Ruijin Ran, Xin Jin, Xiaohong Zhu
Medicine.2022; 101(39): e30877. CrossRef - Emerging roles of selenium on metabolism and type 2 diabetes
Jiuxiang Zhao, Hong Zou, Yanling Huo, Xiaoyi Wei, Yu Li
Frontiers in Nutrition.2022;[Epub] CrossRef - Selenium: Role in preserving and improving health and preventing disease
Goran Belojević
Galenika Medical Journal.2022; 1(4): 90. CrossRef - A comprehensive review on the neuropathophysiology of selenium
Mohammad Naderi, Pankaj Puar, Mahtab Zonouzi-Marand, Douglas P. Chivers, Som Niyogi, Raymond W.M. Kwong
Science of The Total Environment.2021; 767: 144329. CrossRef - Dietary selenium intake and risk of hospitalization for type 2 diabetes in the Moli-sani study cohort
Marco Vinceti, Marialaura Bonaccio, Tommaso Filippini, Simona Costanzo, Lauren A. Wise, Augusto Di Castelnuovo, Emilia Ruggiero, Mariarosaria Persichillo, Chiara Cerletti, Maria Benedetta Donati, Giovanni de Gaetano, Licia Iacoviello
Nutrition, Metabolism and Cardiovascular Diseases.2021; 31(6): 1738. CrossRef - A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies
Marco Vinceti, Tommaso Filippini, Lauren A. Wise, Kenneth J. Rothman
Environmental Research.2021; 197: 111210. CrossRef - Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity
Dominika Radomska, Robert Czarnomysy, Dominik Radomski, Anna Bielawska, Krzysztof Bielawski
Nutrients.2021; 13(5): 1649. CrossRef - Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship With Diseases
Rannapaula Lawrynhuk Urbano Ferreira, Karine Cavalcanti Maurício Sena-Evangelista, Eduardo Pereira de Azevedo, Francisco Irochima Pinheiro, Ricardo Ney Cobucci, Lucia Fatima Campos Pedrosa
Frontiers in Nutrition.2021;[Epub] CrossRef - Ferroptosis and Its Potential Role in Metabolic Diseases: A Curse or Revitalization?
Jia-Yue Duan, Xiao Lin, Feng Xu, Su-Kang Shan, Bei Guo, Fu-Xing-Zi Li, Yi Wang, Ming-Hui Zheng, Qiu-Shuang Xu, Li-Min Lei, Wen-Lu Ou-Yang, Yun-Yun Wu, Ke-Xin Tang, Ling-Qing Yuan
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Selenium Intake and Glycemic Control in Young Adults With Normal-Weight Obesity Syndrome
Acsa de Castro Santos, Anna Flavia Ferreira Passos, Luciana Carla Holzbach, Cristiane Cominetti
Frontiers in Nutrition.2021;[Epub] CrossRef - Development and Therapeutic Potential of Selenazo Compounds
Ana Carolina Ruberte, Carmen Sanmartin, Carlos Aydillo, Arun K. Sharma, Daniel Plano
Journal of Medicinal Chemistry.2020; 63(4): 1473. CrossRef - Selenium in thyroid disorders — essential knowledge for clinicians
Kristian Hillert Winther, Margaret Philomena Rayman, Steen Joop Bonnema, Laszlo Hegedüs
Nature Reviews Endocrinology.2020; 16(3): 165. CrossRef - Safety of selenium‐enriched biomass of Yarrowia lipolytica as a novel food pursuant to Regulation (EU) 2015/2283
Dominique Turck, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch‐Ernst, John Kearney, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri, Marc
EFSA Journal.2020;[Epub] CrossRef The Association of Circulating Selenium Concentrations with Diabetes Mellitus
Xiao-Long Liao, Zhong-Hua Wang, Xiu-Na Liang, Jun Liang, Xue-Biao Wei, Shou-Hong Wang, Wei-Xin Guo
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2020; Volume 13: 4755. CrossRef- A 2018 European Thyroid Association Survey on the Use of Selenium Supplementation in Hashimoto’s Thyroiditis
Kristian Hillert Winther, Enrico Papini, Roberto Attanasio, Roberto Negro, Laszlo Hegedüs
European Thyroid Journal.2020; 9(2): 99. CrossRef - Systems Biology of Selenium and Complex Disease
Huimin Ying, Yan Zhang
Biological Trace Element Research.2019; 192(1): 38. CrossRef - Effectiveness and safety of selenium supplementation for type 2 diabetes mellitus in adults: a systematic review of randomised controlled trials
A. Stróżyk, Z. Osica, J. D. Przybylak, M. Kołodziej, B. M. Zalewski, B. Mrozikiewicz‐Rakowska, H. Szajewska
Journal of Human Nutrition and Dietetics.2019; 32(5): 635. CrossRef - Selenium and Health: An Update on the Situation in the Middle East and North Africa
Sohayla A. Z. Ibrahim, Abdelhamid Kerkadi, Abdelali Agouni
Nutrients.2019; 11(7): 1457. CrossRef
- Increased Expression of PHGDH Under High-Selenium Stress In Vivo
- Clinical Diabetes & Therapeutics
- Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial
- Tae Jung Oh, Jae Myung Yu, Kyung Wan Min, Hyun Shik Son, Moon Kyu Lee, Kun Ho Yoon, Young Duk Song, Joong Yeol Park, In Kyung Jeong, Bong Soo Cha, Yong Seong Kim, Sei Hyun Baik, In Joo Kim, Doo Man Kim, Sung Rae Kim, Kwan Woo Lee, Jeong Hyung Park, In Kyu Lee, Tae Sun Park, Sung Hee Choi, Sung Woo Park
- Diabetes Metab J. 2019;43(3):276-286. Published online December 7, 2018
- DOI: https://doi.org/10.4093/dmj.2018.0051
- 7,059 View
- 99 Download
- 13 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Combination of metformin to reduce the fasting plasma glucose level and an α-glucosidase inhibitor to decrease the postprandial glucose level is expected to generate a complementary effect. We compared the efficacy and safety of a fixed-dose combination of voglibose plus metformin (vogmet) with metformin monotherapy in drug-naïve newly-diagnosed type 2 diabetes mellitus.
Methods A total of 187 eligible patients aged 20 to 70 years, with a glycosylated hemoglobin (HbA1c) level of 7.0% to 11.0%, were randomized into either vogmet or metformin treatments for 24 weeks. A change in the HbA1c level from baseline was measured at week 24.
Results The reduction in the levels of HbA1c was −1.62%±0.07% in the vogmet group and −1.31%±0.07% in the metformin group (
P =0.003), and significantly more vogmet-treated patients achieved the target HbA1c levels of <6.5% (P =0.002) or <7% (P =0.039). Glycemic variability was also significantly improved with vogmet treatment, estimated by M-values (P =0.004). Gastrointestinal adverse events and hypoglycemia (%) were numerically lower in the vogmet-treated group. Moreover, a significant weight loss was observed with vogmet treatment compared with metformin (−1.63 kg vs. −0.86 kg,P =0.039).Conclusion Vogmet is a safe antihyperglycemic agent that controls blood glucose level effectively, yields weight loss, and is superior to metformin in terms of various key glycemic parameters without increasing the risk of hypoglycemia.
-
Citations
Citations to this article as recorded by- Phytochemical analysis and antihyperglycemic activity of Castilleja arvensis
Mónica Aideé Díaz-Román, Juan José Acevedo-Fernández, Gabriela Ávila-Villarreal, Elizabeth Negrete-León, A. Berenice Aguilar-Guadarrama
Fitoterapia.2024; 174: 105839. CrossRef - YAP/TAZ axis was involved in the effects of metformin on breast cancer
Yu Xu, Hongke Cai, Yuanfeng Xiong, Li Tang, Longjiang Li, Li Zhang, Yi Shen, Yongqiang Yang, Ling Lin, Jiayi Huang
Journal of Chemotherapy.2023; 35(7): 627. CrossRef - Diabetes remission: Myth or reality?
Ashok Kumar, ShubhaLaxmi Margekar, Ravi Kumar
Indian Journal of Medical Specialities.2023; 14(1): 3. CrossRef - Analysis of Reports Sent to the Portuguese Pharmacovigilance System and Published Literature Regarding the Safety of Metformin in the Elderly
Beatriz Esteves, Cristina Monteiro, Ana Paula Coelho Duarte
Healthcare.2023; 11(15): 2197. CrossRef - Rapid prediction method of α-Glycosidase inhibitory activity of Coreopsis tinctoria extract from different habitats by near infrared spectroscopy
Xiaogang He, Xiang Han, Jiaping Yu, Yulong Feng, Ganghui Chu
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy.2022; 268: 120601. CrossRef - Insulin autoimmune syndrome in patients with type 2 diabetes: A report of two cases
Y. Shin, T.J. Oh, S.H. Choi, H.C. Jang
Diabetes & Metabolism.2021; 47(1): 101115. CrossRef - Efficacy and Safety of Treatment with Quadruple Oral Hypoglycemic Agents in Uncontrolled Type 2 Diabetes Mellitus: A Multi-Center, Retrospective, Observational Study
Jun Sung Moon, Sunghwan Suh, Sang Soo Kim, Heung Yong Jin, Jeong Mi Kim, Min Hee Jang, Kyung Ae Lee, Ju Hyung Lee, Seung Min Chung, Young Sang Lyu, Jin Hwa Kim, Sang Yong Kim, Jung Eun Jang, Tae Nyun Kim, Sung Woo Kim, Eonju Jeon, Nan Hee Cho, Mi-Kyung Ki
Diabetes & Metabolism Journal.2021; 45(5): 675. CrossRef - Quantifying Remission Probability in Type 2 Diabetes Mellitus
Sanjay Kalra, Ganapathi Bantwal, Nitin Kapoor, Rakesh Sahay, Saptarshi Bhattacharya, Beatrice Anne, Raju A Gopal, Sunil Kota, Ashok Kumar, Ameya Joshi, Debmalya Sanyal, Mangesh Tiwaskar, Ashok Kumar Das
Clinics and Practice.2021; 11(4): 850. CrossRef - The effect of voglibose on metabolic profiles in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of clinical trials
Peyman Nowrouzi-Sohrabi, Reza Tabrizi, Shahla Rezaei, Fatemeh Jafari, Kamran Hessami, Mehdi Abedi, Mohammad Jalali, Pedram Keshavarzi, Saeed Shahabi, Ali Asghar Kolahi, Kristin Carson-Chahhoud, Amirhossein Sahebkar, Saeid Safiri
Pharmacological Research.2020; 159: 104988. CrossRef - Role of Intestinal Microbiota in Metabolism of Voglibose In Vitro and In Vivo
Mahesh Raj Nepal, Mi Jeong Kang, Geon Ho Kim, Dong Ho Cha, Ju-Hyun Kim, Tae Cheon Jeong
Diabetes & Metabolism Journal.2020; 44(6): 908. CrossRef - Response: Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes metab J 2019;43;276-86)
Tae Jung Oh, Sung Hee Choi
Diabetes & Metabolism Journal.2019; 43(4): 547. CrossRef - Letter: Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2019;43;276-86)
Hannah Seok, Tae Seo Sohn
Diabetes & Metabolism Journal.2019; 43(4): 545. CrossRef
- Phytochemical analysis and antihyperglycemic activity of Castilleja arvensis
- Response: The Effect of an Angiotensin Receptor Blocker on Arterial Stiffness in Type 2 Diabetes Mellitus Patients with Hypertension (Diabetes Metab J 2011;35:236-42)
- Ji Hyun Kim, Su Jin Oh, Jung Min Lee, Eun Gyoung Hong, Jae Myung Yu, Kyung Ah Han, Kyung Wan Min, Hyun Shik Son, Sang Ah Chang
- Diabetes Metab J. 2011;35(4):429-430. Published online August 31, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.4.429
- 3,749 View
- 27 Download
- The Effect of an Angiotensin Receptor Blocker on Arterial Stiffness in Type 2 Diabetes Mellitus Patients with Hypertension
- Ji Hyun Kim, Su Jin Oh, Jung Min Lee, Eun Gyoung Hong, Jae Myung Yu, Kyung Ah Han, Kyung Wan Min, Hyun Shik Son, Sang Ah Chang
- Diabetes Metab J. 2011;35(3):236-242. Published online June 30, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.3.236
- 28,961 View
- 35 Download
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Hypertension and type 2 diabetes mellitus are major risk factors for cardiovascular disease. This study analyzed the changes in central aortic waveforms and pulse wave velocity as well as related parameters after treatment with valsartan, an angiotensin II type 1 receptor blocker, in patients with type 2 diabetes and hypertension.
Methods We used pulse wave analysis to measure central aortic waveform in a total of 98 subjects. In 47 of these patients, pulse wave velocity measurements were obtained before and after 12 weeks of treatment with valsartan.
Results In the central aortic waveform analysis, the aortic pulse pressure and augmentation index were significantly decreased after valsartan treatment, as was the aortic pulse wave velocity. Factors contributing to the improvement in pulse wave velocity were the fasting blood glucose and haemoglobin A1c levels.
Conclusion Short-term treatment with valsartan improves arterial stiffness in patients with type 2 diabetes and hypertension, and the glucose status at baseline was associated with this effect.
-
Citations
Citations to this article as recorded by- Mechanisms underlying the blood pressure‐lowering effects of empagliflozin, losartan and their combination in people with type 2 diabetes: A secondary analysis of a randomized crossover trial
Rosalie A. Scholtes, Charlotte M. Mosterd, Anne C. Hesp, Mark M. Smits, Hiddo J. L. Heerspink, Daniël H. van Raalte
Diabetes, Obesity and Metabolism.2023; 25(1): 198. CrossRef - Distinct effects of losartan and atenolol on vascular stiffness in Marfan syndrome
Ami B Bhatt, J Stewart Buck, Jonah P Zuflacht, Jessica Milian, Samoneh Kadivar, Kimberlee Gauvreau, Michael N Singh, Mark A Creager
Vascular Medicine.2015; 20(4): 317. CrossRef - The impact of angiotensin receptor blockers on arterial stiffness: a meta-analysis
Feng Peng, Hongming Pan, Bin Wang, Jinxiu Lin, Wenquan Niu
Hypertension Research.2015; 38(9): 613. CrossRef - Arterial stiffness in atherosclerotic renovascular hypertension
Ljiljana Fodor, Vedran Premužić, Vanja Ivković, Dražen Perkov, Mario Laganović, Tajana Željković Vrkić, Živka Dika, Marijana Živko, Bojan Jelaković
Journal of Hypertension.2014; 32(11): 2238. CrossRef - Improvement of arterial wall characteristics by the low-dose fluvastatin and valsartan combination in type 1 diabetes mellitus patients
Vedran Savić, Barbara Eržen, Miodrag Janić, Mojca Lunder, Maja Boncelj, Karin Kanc, Andrej Janež, Mišo Šabovič
Diabetes and Vascular Disease Research.2013; 10(5): 420. CrossRef - The association between regional arterial stiffness and diabetic retinopathy in type 2 diabetes
Won Jun Kim, Cheol-Young Park, Se Eun Park, Eun Jung Rhee, Won Young Lee, Ki Won Oh, Sung Woo Park, Sun Woo Kim, SuJeong Song
Atherosclerosis.2012; 225(1): 237. CrossRef - Letter: The Effect of an Angiotensin Receptor Blocker on Arterial Stiffness in Type 2 Diabetes Mellitus Patients with Hypertension (Diabetes Metab J 2011;35:236-42)
Chul-Hee Kim
Diabetes & Metabolism Journal.2011; 35(4): 427. CrossRef
- Mechanisms underlying the blood pressure‐lowering effects of empagliflozin, losartan and their combination in people with type 2 diabetes: A secondary analysis of a randomized crossover trial
- Risk Factors for Early Development of Macrovascular Complications in Korean Type 2 Diabetes.
- Hae Ri Lee, Jae Myung Yu, Moon Gi Choi, Hyung Joon Yoo, Eun Gyoung Hong
- Korean Diabetes J. 2009;33(2):134-142. Published online April 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.2.134
- 2,221 View
- 23 Download
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The average duration of diabetes and predictive factors of macrovascular complications in Korean diabetic patients remain to be elucidated. This study examines the average duration of diabetes up to the onset of macrovascular complications and clinically important factors of early development of these complications in Korean type 2 diabetic patients. METHODS: Clinical characteristics in type 2 diabetics with (n = 121) and without macrovascular complications (n = 115) were analyzed. In addition, early onset (< or = 5 years, n = 54) and late onset groups (> 5 years, n = 67) were compared, as were the clinical characteristics between male and female patients in the macrovascular complications group. RESULTS: The average duration of diabetes was 8.7 +/- 7.8 years in the macrovascular complications group. Average age, systolic and diastolic blood pressures and smoking history were all higher in the macrovascular complications group than the control group. However, HbA1c levels and prevalence of microvascular complications were higher in the controls. Average age was lower in the early onset group and many more patients of that group had a smoking history. In the analysis based on sex, marcrovascular complications developed earlier in male patients. In addition, the prevalence of family history of diabetes was higher in males and 77.8% of male patients had a smoking history (female: 3.4%). CONCLUSION: Our study confirms that older age, high blood pressure and smoking history are major risk factors for the development of macrovascular complications. Moreover, a smoking history in males can be both risk and predictive factors for earlier development of macrovascular complications in Korean type 2 diabetic patients. We also found that several clinical characteristics including age, family history of diabetes, hypertension and smoking history, vary between the sexes, and these findings can provide useful indices for the prevention of macrovascular complications. -
Citations
Citations to this article as recorded by- Impact of new-onset diabetes on clinical outcomes after ST segment-elevated myocardial infarction
Ji-Yeoun Seo, Jin-Sun Park, Kyoung-Woo Seo, Hyoung-Mo Yang, Hong-Seok Lim, Byoung-Joo Choi, So-Yeon Choi, Myeong-Ho Yoon, Gyo-Seung Hwang, Seung-Jea Tahk, Joon-Han Shin
Scandinavian Cardiovascular Journal.2019; 53(6): 379. CrossRef - Associations Between the Continuity of Ambulatory Care of Adult Diabetes Patients in Korea and the Incidence of Macrovascular Complications
Young-Hoon Gong, Seok-Jun Yoon, Hyeyoung Seo, Dongwoo Kim
Journal of Preventive Medicine and Public Health.2015; 48(4): 188. CrossRef - Relationship of Daily Activity and Biochemical Variables in the Elderly with Diabetes Mellitus
Ki-Wol Sung
Journal of Korean Academy of Nursing.2011; 41(2): 182. CrossRef - Epidemiology of Micro- and Macrovascular Complications of Type 2 Diabetes in Korea
Jung Hee Kim, Dae Jung Kim, Hak Chul Jang, Sung Hee Choi
Diabetes & Metabolism Journal.2011; 35(6): 571. CrossRef
- Impact of new-onset diabetes on clinical outcomes after ST segment-elevated myocardial infarction
- Pedometer-Determined Physical Activity in Type 2 Diabetes in Korea.
- Sang Ah Chang, Jung Min Lee, Tae Seo Sohn, Hyun Shik Son, Sung Woo Park, Sei Hyun Baik, Jae Myung Yu, Yeon Ah Sung, Chul Woo Ahn, Kyung Wan Min, Kyung Ah Han
- Korean Diabetes J. 2007;31(1):83-88. Published online January 1, 2007
- DOI: https://doi.org/10.4093/jkda.2007.31.1.83
- 1,747 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Walking is a popular, convenient and relatively safe form of exercise. However, there is few objective data for walking exercise. The aim of this study was to evaluate pedometer-determined physical activity defined as steps/day in type 2 diabetes mellitus. Therefore, it could be the basic data for programming walking exercise in diabetes mellitus. METHODS: Participants with type 2 diabetes who visited in 6 university hospitals on February, 2006 in Seoul and Kyung-gi area were recruited. The participants were asked their ambulatory activity with the given pedometer and calorimeter for 1 week. Total 240 (Male 122, Female 118) subjects who walked above 1000 steps/day were analyzed. We also collected their biochemical data from the medical records. RESULTS: Participants took 8532 +/- 4130 steps for day (step/day) and energy expenditure were 320 +/- 161 Cal/day. Steps/day was not significantly different between male and female, but energy expenditure was higher in male than female ( P < 0.05). Steps/day was significantly lower in obese patients than non-obese patients (P < 0.001). BMI (r = -0.325, P < 0.001), waist circumference (r = -0.287, P < 0.001), triglyceride (r = 0.164, P < 0.018) showed significant inverse correlation with steps/day, but BUN (r = 0.165, P = 0.019) and HDL-cholesterol (r = 0.164, P = 0.018) were positive correlated with steps/day significantly. BMI (r = -0.14, P < 0.032) and cholesterol (r = -0.139, P < 0.041) showed significantly inverse correlation with energy expenditure and BUN (r = 0.187, P = 0.008) and HDL cholesterol (r = 0.145, P < 0.037) positively correlated with energy expenditure. Pedometer-determined steps/day was positively associated with energy expenditure (r2 = 0.824, P < 0.001). CONCLUSION: This study showed the objective quantification of physical activity measured by simple and inexpensive pedometers. It could be used to recommend walking exercise since the practitioners can estimate steps/day for required energy expenditure.
- Evaluation of the Indicator Test(NeurocheckTM) in the Diagnosis of Peripheral Neuropathy among Type 2 Diabetic Patients.
- Tae Seo Sohn, Hyun Shik Son, Jae Myung Yu, Bong Soo Cha, Kyung Wan Min, Sei Hyun Baik
- Korean Diabetes J. 2005;29(3):247-253. Published online May 1, 2005
- 877 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Eighty-five percent of the lower-limb amputations that are done for patients with diabetes are preceded by foot ulceration, and this suggests that prevention and the appropriate management of foot lesions are of paramount importance. Ulceration is caused by several factors acting together, but they are particularly caused by neuropathy. Various aspects of the neurovascular function can be evaluated with specialized tests, but these tests have generally not been well standardized and they have limited clinical utility. A new indicator test(NeurocheckTM) that utilizes the water-induced color change of a cobaltII compound from blue to pink has been introduced. The aim of the present study was to evaluate this new indicator test in the diagnosis of peripheral neuropathy among type 2 diabetic patients. METHOD: This study included 124 diabetic patients(45 men and 79 women) who were recruited from 5 diabetic centers in Korea. The presence of diabetic neuropathy was diagnosed by nerve conduction study. The degree of the patient's symptom was checked as the total symptom score(TSS). Autonomic sudomotor neuropathy was assessed by means of the new indicator test(NeurocheckTM). The degree of color change in 10 minutes was assessed as a complete color change, an incomplete color change or no color change. RESULTS: Of the 124 diabetics patients we investigated, 109 patients were proven to have peripheral neuropathy by nerve conduction study. Autonomic sudomotor neuropathy by NeurocheckTM was diagnosed in 94 patients with peripheral neuropathy(86.2%) and in 6 patients(40%) without peripheral neuropathy. The overall measure of agreement between NeurocheckTM and the electrodiagnostic test was 0.3673(0.1547, 0.58). The sensitivity and specificity of NeurocheckTM was higher in women(91.2% and 63.6%, respectively) than in men(78.0% and 50.0%, respectively). The measure of agreement in women was 0.5093(0.2396, 0.9601) and in the men it was 0.1567(-0.1423, 0.4588). CONCLUSION: The new indicator test has a high sensitivity for the diagnosis of peripheral neuropathy among diabetic patients, especially in women. It is likely that the new indicator test is useful clinically as a screening and diagnostic tool for diabetic neuropathy. Since the specificity of the test is somewhat low, the patients with a high total symptom score and who are without sudomotor neuropathy may need further diagnostic evaluation on neuropathy
- Two Cases of Hyperamylasemia not Aassociated with Acute Pancreatits in Non-ketotic Hyperosmolar Syndrome.
- Jong Hyung Choi, Doo Man Kim, Han Su Cho, Ki Sung Lee, Ji Young Seo, Hyun Kyoo Kim, Cheol Soo Choi, Sung Hee Ihm, Jae Myung Yu, Moon Ki Choi, Hyung Joon Yoo, Sung Woo Park
- Korean Diabetes J. 2000;24(5):614-618. Published online January 1, 2001
- 1,199 View
- 16 Download
-
 Abstract
Abstract
- The serum amylase level is widely used as a screening test for acute pancreatitis and rises also in a wide variety of diseases involving the pancreas, salivary glands, intestines, liver, genitourinary tract, and lung, in metabolic aberrations such as diabetic ketoacidosis, and even during normal pregnancy. Although it is commonly assumed that the diseased organ is releasing amylase into the serum, in many conditions the precise relationship between the hyperamylasemia and the condition is not clear. Serum amylase is abnormally elevated in more than 60% of patients with diabetic ketoacidosis, but increased pancreatic enzyme activity, even in combination with abdominal pain, should not be diagnosed as acute pancreatitis. In nonketotic hyperosmolar syndrome, elevated serum amylase level without pancreatitis has not been reported. Nonketotic hyperosmolar syndrome is usually a complcation of type 2 DM and characterized by severe hyperosmolarity (serum osmolality> or =320 mOsm/L), hyperglycemia (serum glucose> or = 600 mg/dL) and dehydration. We experienced two cases of nonketotic hyperosmolar syndrome with elevated serum amylase. Serum amylase level was 1556 U/L in first case, 229 U/L in second case. Two patients did not complain of abdominal pain, nausea, vomiting and abdomen CT with enhancement showed the normal pancreases.

 KDA
KDA
 First
First Prev
Prev





